Revitalizing US Biomanufacturing to Strengthen the Global Supply and Security of Antibiotics
Renewed US investment in domestic antibiotic production is essential to ensure supply chain resilience and safeguard global health security.

This is reprinted with permission from The Bridge, Summer 2025, pgs. 48–55. To view a downloadable PDF version of this essay, please click here.
Misti Ushio is a managing partner at Digitalis Ventures. Barry C. Buckland is executive director at the National Institute for Innovation in Manufacturing Biopharmaceuticals (NIIMBL).
Antibiotics have significantly improved life expectancy and overall public health for over 80 years. The primary reasons antibiotics are critical for human health include treating life-threatening infection, supporting advanced surgical medicine, protecting public health via control of widespread infection, and impacting health economics by reducing hospital stay, prolonged illness, etc. Penicillin, the first antibiotic derived from a natural organism, was introduced in 1942 and revolutionized medicine by effectively treating infections such as pneumonia, sepsis, and syphilis. It is estimated that penicillin alone has saved approximately 200 million lives, and its discovery paved the way for further advancements in antibiotics that have collectively saved hundreds of millions more lives to date (University of Sheffield 2021).
Today, however, the global antibiotic production landscape poses risks to global health security and equitable access to key medicines. The production of antibiotic active pharmaceutical ingredients (APIs) has become concentrated in a handful of countries; nearly 70% of the manufacturing sites for a representative shortlist of 40 antibiotic APIs are located in two countries, India and China (with the majority in China). More concerning is that the United States no longer has any significant fermentation manufacturing capabilities to produce antibiotic APIs onshore. This signals potential vulnerabilities in the supply chain and disparities in global health resilience.
Numerous opportunities exist to leverage policy and technology to ensure a more resilient, reliable supply of antibiotics. There is a need to increase the discussion about antibiotic security between government, manufacturing, and technology development to create expanded partnership and coordination among all stakeholders instead of local decision-making based only on cost. If global redistribution of antibiotic production is a health priority, the tools of synthetic biology can be applied to improve production efficiency and reduce costs while improving global health security and access. Translating this into manufacturing would require collaboration among governments, industry leaders, and research institutions to establish a robust, secure, and sustainable antibiotic manufacturing infrastructure that benefits all nations. New manufacturing facilities also invite the opportunity to apply other innovations such as continuous culture, extensive use of robotics, automation, and application of AI to improve process performance. By embracing these measures, the international community can better safeguard public health worldwide.
It is the opinion of the authors that 1) the United States and other countries should proactively discuss how to increase antibiotic security via collaboration between government, manufacturing, and technology development to create partnership and coordination among all stakeholders instead of local decision-making based only on cost, and 2) the United States and other countries should take steps to prevent the cycle that has occurred for antibiotic security from being repeated with antibody security.
Penicillin History
1/ Discovery and Development of Penicillin, the World’s First Antibiotic
In 1928, the bacteriologist Alexander Fleming discovered penicillin by observing that a stray spore of a mold, subsequently identified as Penicillium notatum, settled on a petri dish that had a bacterial culture of staphylococcus on it. This mold colony was surrounded by a zone of growth inhibition and lysis, indicating that the mold was killing the bacteria. Fleming understood the potential significance of this and preserved the penicillium culture over many years. However, subsequent progress was delayed by the instability of penicillin.
Beginning in 1939, a group at Oxford University led by Howard G. Florey, an Australian pathologist, and Ernst Chain, a chemist who had fled Nazi Germany, developed an assay, found a way of producing penicillin in surface culture, made enough for preclinical studies, and solved the stability issue partially via lyophilization. Excitement grew as soon as the preclinical studies were completed.
Because of the devastating wartime situation in England, two members of the Oxford team traveled to the United States to get help. Florey and Norman G. Heatley arrived in New York on July 2, 1941. There followed a remarkable collaboration between academia, government, and industry, resulting in supplies of penicillin being made available via submerged fermentation at large scale by D-Day on June 6, 1944. In the words of Florey and the Oxford team: “Too high a tribute cannot be paid to the enterprise and energy with which the American manufacturing firms tackled the large-scale production of the drug. Had it not been for their efforts, there would certainly not have been sufficient penicillin by D-day in Normandy” (Journal of the Royal Society of Medicine, 1949).
Alexander Fleming, Ernst Chain, and Howard Florey were awarded the Nobel Prize in Physiology and Medicine in 1945 to broad acclaim (Nobel Prize.org 2025). The Nobel Prize acknowledged not only a monumental scientific discovery but also a great contribution to the emerging field of bioprocess engineering.

Sir Alexander Fleming, 1943

Seven of the early researchers on penicillin. (Back row, left to right) S. Waksman, H. Florey, J. Trefouel, E. Chain, A. Gratia, (front row left to right) P. Fredericq & Maurice Welsch.

The National Institute of Health (NIH) in Bethesda, Maryland, 1940
The introduction of penicillin occurred during World War II because of a remarkable collaboration between universities, government, and the pharmaceutical industry. For biomanufacturing of pharmaceuticals, this effort was the equivalent of the Manhattan Project. A number of companies were involved in this effort, including Merck, based in Rahway, New Jersey, and Pfizer, which was then a small company producing citric acid by fermentation based in Brooklyn, NY. Penicillin was the first antibiotic developed, and it was closely followed by streptomycin.
The great success of penicillin helped trigger the development of many other antibiotics widely prescribed to this day, and the penicillin family of beta lactam antibiotics is still the most commonly prescribed (CDC 2019–2023). In 2023, the prescription rate for penicillins was 182 per 1,000 population in the United States, 55% higher than the second most prescribed class of antibiotics, cephalosporins (CDC 2019–2023).
2/ Biomanufacturing of Penicillin
Various semisynthetic antibiotics are based on penicillin (amoxicillin, ampicillin, dicloxacillin, and penicillin V) and are manufactured via several steps. Semisynthetic means that the base molecule is produced via fermentation and then chemically or enzymatically modified to obtain the maximum therapeutic effect. An example is given here for ampicillin (see table 1 below).
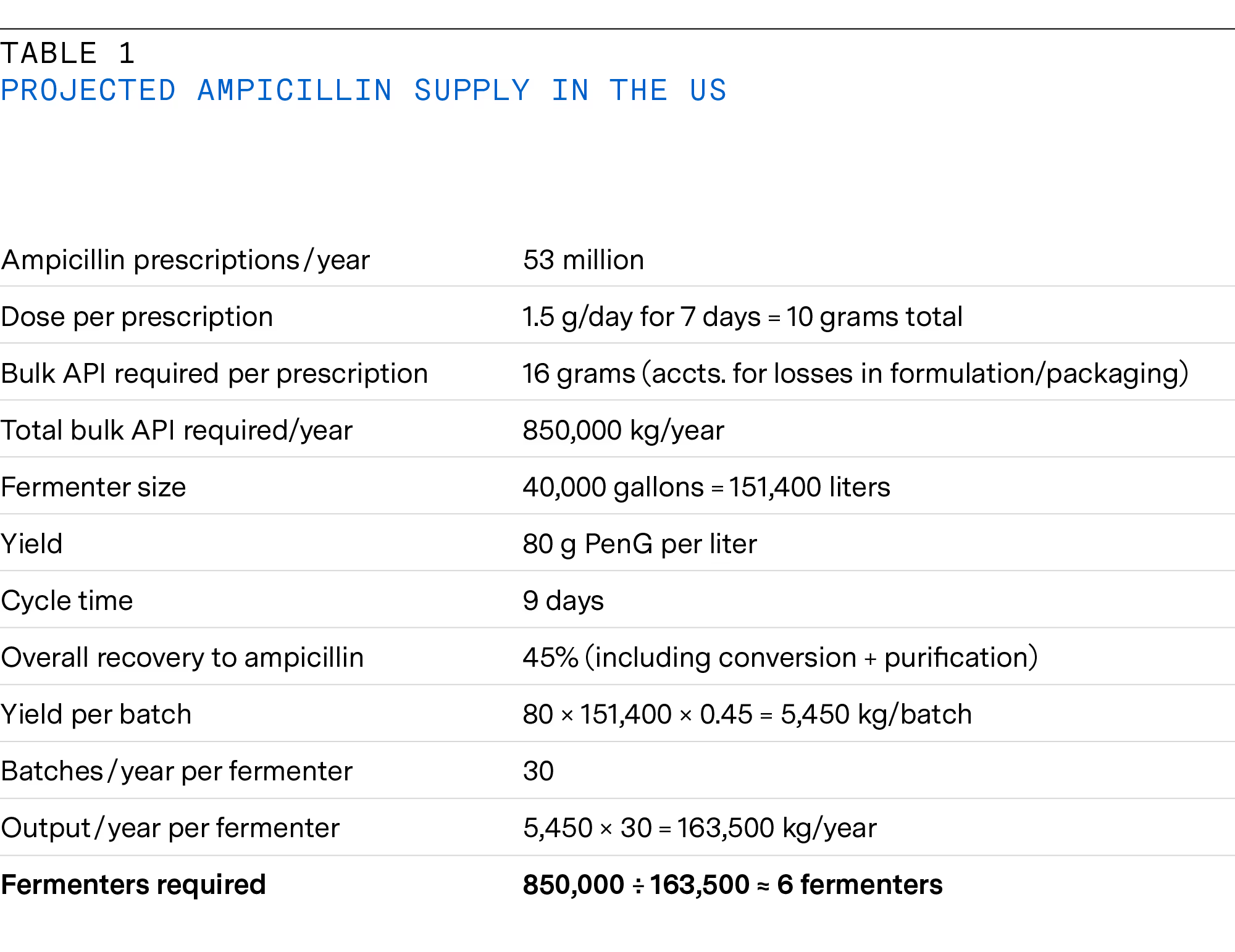
The process begins with the fermentation of the mold Penicillium chrysogenum, which produces penicillin G (benzylpenicillin). Typically, fermentations are run in the fed-batch mode at the 75 to 150 thousand liter scale and last for around eight days, with a final product concentration of more than 40 grams/liter. During harvest, penicillin G is extracted from the culture broth through solvent extraction methods followed by purification processes such as crystallization, chromatography, or filtration to obtain pure penicillin G.
The purified penicillin G is then converted to ampicillin by enzymatic and chemical modifications.
Deacylation: The benzyl group of penicillin G is removed to create a penicillin intermediate.
Acylation: The penicillin nucleus is then acylated with the appropriate amino acid or other acylating agents (such as 4-aminobenzylpenicillin) to form ampicillin. This reaction alters the side chain, transforming penicillin G into ampicillin. The newly formed ampicillin undergoes further purification processes to remove any unreacted materials and by-products. This can include additional crystallization, precipitation, or chromatographic techniques.
After purification, ampicillin can be formulated into various forms, including tablets, capsules, and injectable solutions. This process allows for the efficient transformation of naturally occurring penicillin into a semi-synthetic antibiotic with a broader spectrum of activity, making ampicillin effective against various bacterial infections.
Over time, improvements have been made to penicillin manufacturing, including increasing productivity of the producing penicillium strain and optimizing both nutrient feeding strategies and mixing conditions. Downstream purification methods, such as extensive solvent recycling, have also been improved. Semisynthetic molecules have boosted the antimicrobial efficacy of virtually all other microbial-derived antibiotics, including erythromycin, tetracycline, and cephalosporins. The parent molecule in each case is manufactured by deep tank fermentations (see figures 1 & 2 below). The same microbial engineering strategy can be applied in the same way as described here for penicillin.

Figure 1. Dr. Selman A. Waksman, J.H. Holcomb, Jr., and Dr. E.J. Nolan in front of a fermenter used in Merck’s streptomycin plant, December 1945. Photo credit: Merck Archives.

Figure 2. Large containers of mold and growth medium with submerged fermenters. Record Group 208, Records of the Office of War Information, 1926–1951, Still Pictures and Records Section, 208-SAI-1-18 Antibiotics, National Archives, College Park, Md.
Today there are many examples of antibiotics manufactured via fermentation on a very large scale:
Penicillin
Typically produced by the fermentation of Penicillium chrysogenum.
Cephalosporins
Similar to penicillins, they are produced by the fermentation of the fungus Cephalosporium acremonium.
Tetracyclines
These broad-spectrum antibiotics are produced by the fermentation of streptomyces bacteria, primarily Streptomyces aureofaciens.
Erythromycin
Made through the fermentation of Streptomyces erythreus.
Streptomycin
Produced by the bacterium Streptomyces griseus.
Gentamicin
This is produced from the fermentation of Micromonospora purpurea and other micromonospora species.
Vancomycin
Derived from the bacterium Streptomyces orientalis.
Rifamycin
Produced by Streptomyces mediterranei.
Expansion of Antibiotic Development and the Geographical Shift of Antibiotic Manufacturing Capacity over the Past 80 Years
“The Marcy Avenue penicillin plant was 95% completed by the end of February [1944] and deep tank fermentation was initiated. Working 24 hours a day, seven days a week, the increase in penicillin production was dramatic. During the fall months, one day’s production of penicillin often exceeded the entire production of 1943” (Ginsberg 2008).
The immense success of penicillin drove a concerted effort to produce antibiotics at greater scale. In the 1940s, pharmaceutical companies invested in large-scale fermentation capacity to manufacture antibiotic APIs and finished products in the United States. Pfizer, for example, established a significant share of early penicillin supply by starting up a manufacturing facility in Brooklyn in March 1944 with 14 large fermenters, 7500 gallons (~28,000 liters) each (American Chemical Society 2024). In this facility, Pfizer could produce penicillin so that sufficient quantities were available in time for the D-Day landings. This was the first large-scale penicillin facility in the world, and it was ultimately designated a National Historic Chemical Landmark by the American Chemical Society on June 12, 2008 (American Chemical Society 2024).
By 1984, many major pharmaceutical companies had scaled and leveraged onshore fermentation capacity to position the United States as a key supplier to the rest of the world. The average facility consisted of 10 to 20 stainless steel fermenters, approximately 75 to 150,000 liters each. Assuming an average of 2.3 million liters total capacity per company suggests an estimated total of 18 million liters of capacity at that time.
However, since then, pharmaceutical companies have steadily outsourced and shifted antibiotic manufacturing to other countries, largely driven by opportunities to reduce costs and avoid capital investment. As of 2021, the leading exporters of antibiotic APIs were China, Italy, India, and Switzerland (Yang et al. 2024). When considering only antibiotic finished products, the top exporters were Italy, Canada, India, and Germany (Yang et al. 2024). The United States is noticeably missing from both lists and does not meaningfully contribute to either antibiotic API or antibiotic production, making the market heavily reliant on exports from other countries (see table 2).

Over the past 20+ years, China has steadily grown into the leading exporter of antibiotic APIs and continues to gain market share of antibiotic medicines as well. In 2021, China-based manufacturers exported 44.5% of total antibiotic APIs exports (up from 9.0% in 2002) and 18.9% of total antibiotic medicines globally (Yang et al. 2024). India has also increased its antibiotic medicine production significantly, though it sources over 82% of APIs from China (Yang et al. 2024).
Today in the United States, USAntibiotics has succeeded in becoming a producer of amoxicillin but outsources the supply of the API (which is made by large-scale fermentation abroad), creating a level of vulnerability in the supply chain.
Importance of Bringing Antibiotic Manufacturing Back to the United States
There are many reasons why it’s crucial to bring antibiotic manufacturing back to the United States, including:
Significant Impact on Human Health
Since their discovery, antibiotics are estimated to have saved at least 200 million lives (University of Sheffield 2021). And they continue to be an essential tool for combating infections. In 2023, there were 252 million prescriptions for antibiotics distributed in the U.S. alone (CDC 2019–2023). Yet, the amount of antibiotics manufactured in the United States has dwindled to a concerningly low level; 92% of the 111 most-prescribed antibiotics have no US source as of 2021 (Miller 2021).
Protect National Security
Ensuring the manufacturing of antibiotics in the United States is a national security issue. Maintaining some level of specialized fermentation manufacturing capacity onshore enhances domestic capacity to respond to public health emergencies and decreases vulnerability to global supply chain disruptions and geopolitical tensions. There have already been several incidents in recent years that have highlighted the fragility of the antibiotic supply chain. During the COVID-19 pandemic, India limited exports of antibiotics tinidazole and erythromycin, among other drugs, due to dwindling supply of API resulting from the temporary closure of Chinese manufacturing facilities (Ellis-Petersen 2020). Further, in 2017, there was a global shortage of piperacillin-tazobactam and benzathine penicillin because a single factory in China shut down; only three API manufacturers for these products remain, all of which are located in China (Yang et al. 2024).
Policy: Precedent, Opportunity, and Challenges
There have been several instances of countries implementing policies to ensure access to key products; it is the opinion of the authors that similar policies should be designed for antibiotic APIs. In what follows, we outline examples of how the United States and other countries have created policy and economic incentives to increase onshore manufacturing and secure access to and independence of key products, identify opportunities for the government to support bringing antibiotics back onshore, and discuss potential challenges in doing so.
Precedent
United States CHIPS Act
The CHIPS and Science Act strengthens US national security by reducing dependence on foreign semiconductor manufacturing, particularly from geopolitical rivals like China. The act provides $52.7 billion in funding to boost domestic semiconductor production, research, and workforce development, ensuring that the United States maintains a secure and resilient supply of critical microchips used in defense, infrastructure, and consumer technology (US Congress 2022).
India Production Linked Incentive
India’s Production Linked Incentive (PLI) scheme enhances antibiotic security by promoting domestic manufacturing of APIs, key starting materials (KSMs), and drug intermediates (Department of Pharmaceuticals, Government of India 2020). This reduces India’s reliance on imports, particularly from China, which currently dominates global API production. By boosting domestic API production, the PLI scheme plays a crucial role in securing India's antibiotic supply, protecting public health, and reducing strategic vulnerabilities in pharmaceutical manufacturing.
United States Project BioShield Partnership with Paratek
In December 2019, the Biomedical Advanced Research and Development Authority (BARDA) partnered with Paratek Pharma to support the development, manufacturing, and procurement of novel antibiotics to treat pulmonary anthrax. This partnership is currently valued at approximately $304 million and has successfully secured manufacturing in the United States, though it took over five years and highlights some of the challenges of bringing back manufacturing infrastructure that has been decommissioned over the past several decades (Paratek Pharmaceuticals 2024).
Opportunity
Implement Economic Incentives
Higher costs to produce antibiotics onshore ultimately drove antibiotic production overseas. Economic incentives such as tax credits and subsidies could motivate pharmaceutical companies to invest in manufacturing capacity back in the United States.
Public-Private Partnerships
Collaboration between the government and the private sector, particularly via government funding, can support and drive innovation in manufacturing capabilities and technology.
Government Procurement & Stockpiling
Guaranteed purchasing agreements from the government or public entities can provide financial stability for antibiotic manufacturers and make investing in fermentation or manufacturing capabilities a more attractive, lower-risk opportunity.
Improve Quality
Access to high-quality antibiotics supports public health. Domestic production of antibiotics provides higher levels of regulatory oversight and control, including more direct inspections to ensure compliance with Good Manufacturing Practices (GMP). While quality is not necessarily dependent on manufacturing location, strict, established regulatory frameworks in the United States could benefit antibiotic quality overall.
Challenges
Opportunity Cost for Pharmaceutical Companies
Pharmaceutical companies have historically prioritized more profitable, chronic disease treatments; antibiotics are prescribed for short durations and generate significantly less revenue compared to other drugs (Dattani 2024). Any new economic incentives need to be meaningful enough to bridge this gap significantly.
Stricter US Environmental Regulations Introduce Manufacturing Costs
High levels of antibiotics in the water supply can increase the rise of antibiotic-resistant pathogens, and to mitigate this, the United States has established regulations on manufacturing waste. Internationally, environmental emissions are largely unregulated, which decreases the cost of production comparatively. However, in 2024, the World Health Organization (WHO) published its first guidance on wastewater and solid waste management in antibiotic manufacturing, which may ultimately bring similar regulations worldwide.
New Technology: Opportunity and Key Considerations
How did the United States go from having approximately 18 million liters of fermenter capacity for making antibiotics in 1984 to next to none today?
The initial technology development had a focus on quality and reducing the cost of goods. The rapid development of biochemical engineering starting in 1940 was driven to a large extent by the opportunity created by very large-scale antibiotic fermentation facilities. Great progress was made by the development of submerged fermentations using low-cost media ingredients and by low-cost purification techniques based on solvent extraction and crystallization as well as the development of sophisticated analytical methods. The bulk price for penicillin was ~$300/kg in 1953 and came down to ~$35/kg by 1980 (Bhattacharyya and Sen 2006).
Due to the cost savings of production overseas, the companies that owned fermentation infrastructure either sold or decommissioned the manufacturing sites and did not replace them. Now there is a minimal amount of fermentation capacity in the United States suitable for manufacturing antibiotics. Given that demand for antibiotics is likely to continue for the foreseeable future, the disinvestment in US fermentation facilities creates a need to rebuild and for investment in new manufacturing technologies.
Advances in synthetic biology represent an opportunity to address global antibiotic security by offering efficient and sustainable manufacturing solutions. By engineering microbial strains and redesigning biosynthetic pathways, synthetic biology facilitates cost-effective production of antibiotics. It accomplishes this by streamlining manufacturing and reducing dependence on complex chemical synthesis, which can increase accessibility and affordability. Namely, it has the potential to simplify multi-step manufacturing processes into a single-tank operation. For example, penicillin is typically purified from fermentation and then enzymatically converted to ampicillin. Similarly, cephamycin C is purified and chemically converted to cefoxitin. These and other examples, including lovastatin and azithromycin, illustrate that synthetic biology could enable processes where both production and conversion occur in the same bioreactor, significantly reducing costs and processing time.
Furthermore, building new facilities and infrastructure creates the opportunity to implement new technologies to increase manufacturing efficiency and reduce costs. Here are several examples for consideration:
- Could synthetic biology be deployed to make ampicillin directly, rather than by first making 6-APA from penicillin G or V and converting it in a separate reaction to the desired ampicillin?
- Could continuous fermentation increase the productivity of currently used fed-batch fermentation, where there is a period of rapid cell growth during which very little product is made followed by a long period of slow growth during which the antibiotic is made? By going to continuous culture with a controlled, slow growth rate, the fermenter productivity can be greatly increased.
- Could the electrical supply come from an adjacent facility using sustainable electricity generation to address the high power demand of an approximately 400 HP per 150,000 liter fermenter? Can current complex media be replaced with low-cost ingredients and sourcing to reduce high waste disposal costs?
- Could the carbon dioxide produced by fermentation be captured and used for a sustainable purpose?
- Could all the solvents required for extraction and crystallization be recycled and used again?
- Could alternative methods of drying be used to reduce the high capital and operating costs of lyophilization?
- Could robotics be used to automate, and could artificial intelligence be applied to contribute to implementing process improvements?
Implications for the Manufacture of Antibodies
The technology for making it practical to manufacture monoclonal antibodies at scale was started in the 1980s and was driven by several innovative companies and universities such as Genentech, Amgen, Biogen, Lonza, Caltech, and MIT. The Engineering Conferences International Cell Culture Engineering Conference (CCE) series was started at this time, and in the beginning many different technical approaches for antibody production were explored. By 1994, there was a convergence of technology, and by the 1994 CCE IV, many companies were using the standard Chinese Hamster Ovary cell line platform with Protein A purification for the capture of their specific antibody. The term “6-pack” was coined to describe the typical manufacturing facility based on six 12,000 liter cell culture bioreactors. Over the past 30 years, there have been continued improvements, most notably the significant increase in product titer that has transformed the 6-pack scale into 2,000 liter bioreactors, but the basic platform remains the same.
The resulting antibody therapies have had dramatic beneficial impacts on human health, including treatment of cancers and chronic inflammatory diseases. For the most part, the manufacturing of these antibody products was initiated in the United States and is now starting to be made in many different countries as the technology transfer is made simpler because there is a common consensus platform. From a strategic national security perspective and quality of goods perspective, we should consider the negative implications of continuing down the same path of local decision-making for supply based primarily on the cost of goods. A national policy should be developed that addresses the strategic importance of domestic antibody production for the security of human health and the national economy.
Conclusion
We suggest the following approaches to start the dialog with key stakeholders to increase awareness, design policy, and fund increased manufacturing capacity:
- Frame antibiotic security as a US national security issue with White House-level acknowledgment that antibiotic dependence on foreign suppliers (mainly China and India) is a threat comparable to energy or semiconductor dependence.
- Design a national defense strategy with the Departments of Defense, Homeland Security, and Health and Human Services recognizing antibiotics as a critical component of infrastructure resilience and assess supply chain vulnerabilities, recommend strategies to restore domestic production, and prioritize public-private partnerships.
- Educate Congress on the state of antibiotic supply chains and discuss legislation similar to the CHIPS and Science Act but for antibiotics, which may include subsidies, tax incentives, investment in next-gen manufacturing technology, and guaranteed government contracts for US antibiotic manufacturing.
- Lead G7 and G20 discussions on global antibiotic resilience, emphasizing that shared dependencies make all nations vulnerable and that, while antibiotic resistance is often in the news, antibiotic supply fragility is not.
Acknowledgments
This work was funded in part by support from the US Department of Commerce, National Institute of Standards and Technology (70NANB21H086). The authors would like to thank Michael Fall for her helpful edits, efforts, and contributions to this article.
References
American Chemical Society. 2024. “New study highlights China’s dominance in global antibiotic supply chains.” EurekAlert!, Feb 21.
Bhattacharyya BK, Sen SK. 2006. “Antibiotics business: A glimpse.” Indian Journal of Biotechnology 5(4):471–76.
CDC (Centers for Disease Control and Prevention). 2019–2023. Outpatient antibiotic prescriptions—all classes. Antimicrobial Resistance & Patient Safety Portal.
US Congress. 2022. CHIPS and Science Act of 2022. Public Law 117-167, Aug 9.
Dattani S. 2024. “What was the Golden Age of Antibiotics, and how can we spark a new one?” Our World in Data, Dec 22. Online at https://ourworldindata.org/golden-age-antibiotics.
Department of Pharmaceuticals, Government of India. 2020. Production Linked Incentive (PLI) Scheme for Promotion of Domestic Manufacturing of Critical Key Starting Materials (KSMs)/ Drug Intermediates and Active Pharmaceutical Ingredients (APIs) in the Country, accessed March 10, 2025. Online at https://pharma-dept.gov.in/schemes/production-linked-incentive-pli-scheme-promotion-domestic-manufacturing-critical-key.
Ellis-Petersen H. 2020. “India limits medicine exports over coronavirus outbreak.” The Guardian, March 4.
Florey H.W. 1949. “In praise of the ‘Devil.’” Journal of the Royal Society of Medicine 42(1):1–5. January. Online at https://doi.org/10.1177/003591574904200101.
Ginsberg J. 2008. Development of Deep-tank Fermentation. Pfizer Inc.
Miller JY. 2021. “Study: US health security at risk because of medicine manufacturing limits.” Olin Business School, Washington University in St. Louis, Aug 5.
Nobel Prize.org. 2025. The Nobel Prize in physiology or medicine 1945. Nobel Prize Outreach 2025, April 29.
Paratek Pharmaceuticals. 2024. “Paratek Pharmaceuticals announces positive efficacy data for NUZYRA as post-exposure prophylaxis of inhalational anthrax, triggering additional procurement under BARDA Project BioShield contract.” GlobeNewswire, March 5.
University of Sheffield. 2021. “Scientists reveal the secrets to penicillin’s longevity.” ScienceDaily, Oct 25.
Yang Y, Husain L, Huang Y. 2024. “China’s position and competitiveness in the global antibiotic value chain: implications for global health.” Globalization and Health 20:87. Online at https://globalizationandhealth.biomedcentral.com/articles/10.1186/s12992-024-01089-x.
COMMUNICATE
To receive periodic updates on our work, sign up below: